Welcome to back2basics
 It is our hope and objective that you will find this and future issues of back2basics sufficiently informative and technically in-depth to save to your electronic library. A PDF format is available by e-mailing us at info@spmorell.com. It is our hope and objective that you will find this and future issues of back2basics sufficiently informative and technically in-depth to save to your electronic library. A PDF format is available by e-mailing us at info@spmorell.com.
In our first issue, we review the technology of pearlescent pigments. This subject matter has more to do with the science of optics than the science of chemistry. The article will help explain how these pigments create the effects we see. We are proud to represent Eckart America for their line of "effect pigments" including aluminum, bronze, and pearl which are widely used in coatings, plastics, and graphic arts.
Pearlescent Pigments – The Science of Optics
The demand for pearlescent pigments is continuously growing in the coatings, plastics, and graphic arts industries. These pigments are considered to be "multi-layer pigments" and are part of the "effect pigments" family which also includes metallics such as aluminum (commonly referred to as silver) and brass (commonly referred to as bronze or gold). While the market for "effect pigments" is much smaller than for organic or inorganic types, they are growing quite rapidly.
End use applications for pearlescent pigments include automotive finishes, cosmetic packaging, house paints, and specialty inks for attention getting packaging design.
Background
The term "pearlescent pigment" derives from natural pearl and mother-of-pearl. These materials comprise layers that reflect some light while remaining translucent enough to allow light to pass further below the surface. Light is reflected back to reach the observer from several depths, including the top layer, creating an appearance of softness and depth.
 Pearlescent, or nacreous pigments, come from either natural (fish scales) or man made sources (metal oxide coated micas, bismuth oxychloride, basic lead carbonate, lead arsenate). Metal oxide coated micas include TiO2 (titanium dioxide) or Fe2O3 (ferric oxide). Pearlescent, or nacreous pigments, come from either natural (fish scales) or man made sources (metal oxide coated micas, bismuth oxychloride, basic lead carbonate, lead arsenate). Metal oxide coated micas include TiO2 (titanium dioxide) or Fe2O3 (ferric oxide).
While conventional and metallic pigments are essentially planar flakes of an opaque reflective material, pearlescent and other effect type pigments use a multi-layer structure to produce internal reflection, refraction, and optical interference.
Pearlescent pigment grades include silver (white), interference colors (yellow, orange, red, violet, blue, green), earth tone colors (bronze, copper, russet), and gold colors (light gold and gold).
Mica or muscovite (potassium aluminum silicate) is a low RI (refractive index), transparent, natural mineral that allows the transmission of light. When these thin platelets (about 500 nm) are coated with high RI metal oxides (ranging in thickness from 60 to 165 nm) they have the ability to reflect light and, thus, pearlescent pigments are created. The face size of these particles range from 1 to 150 microns and the thickness ranges from 0.1 to 3 microns.
Silver White Colors
Pearlescent pigments are thin, smooth, transparent platelets with high indices of refraction. The alignment of these particles in the end product and their particle size (which determines the luster effect - a function of reflectivity) are important characteristics as well. The larger the particle, the greater the luster and the better the orientation of the flake. While small particles don't orient as well, there are more edges to scatter white light which results in higher opacity and a more "satiny" gloss.
TiO2 coated micas produce luster and give a sense of depth due to reflection, transmission, and further reflection from many layers. A luster impression is observed since the eye collects light reflected from platelets at different layers and, therefore, a sense of depth.
 When white light falls on a multilayer pigment, some is reflected at the metal oxide coated surface. Reflection produces a phase shift of half a wavelength. The rest of the light passes through the outer layer and some of the light is again reflected at the surface of the mica. It emerges from the pigment parallel to the first reflected component, but with a different phase relationship which depends on how much further it had to travel through the metal oxide coated layer. When white light falls on a multilayer pigment, some is reflected at the metal oxide coated surface. Reflection produces a phase shift of half a wavelength. The rest of the light passes through the outer layer and some of the light is again reflected at the surface of the mica. It emerges from the pigment parallel to the first reflected component, but with a different phase relationship which depends on how much further it had to travel through the metal oxide coated layer.
Another important consideration is the angle at which the light entered the layer and the wavelength of the light. If the two reflected components are in phase they will appear bright, but colors which are out of phase will be suppressed by interference.
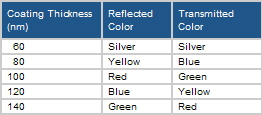 As shown in the table, when mica is coated with TiO2, the silver (white) grades are produced. Varying the thickness of the TiO2 coating will produce the interference colors. As the thickness of the TiO2 layer increases, the reflected color changes from silver (white), to yellow, to red, to blue, and to green. As shown in the table, when mica is coated with TiO2, the silver (white) grades are produced. Varying the thickness of the TiO2 coating will produce the interference colors. As the thickness of the TiO2 layer increases, the reflected color changes from silver (white), to yellow, to red, to blue, and to green.
Interference Colors
Optical principals for pearlescent pigments obey the laws of thin films. Interference colors emanate from light waves originating, or reflecting, from thinly spaced interfaces with varying distances. These light waves interfere with each other and, via the additive process, produce color.
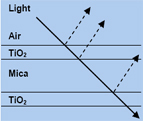 Conventional pigments produce the perceived color due to absorption, or the subtractive process, and reflection of the remaining light waves. In interference colors, the light waves which are reflected back from varying, small, distances interfere with each other and, depending on the wave phase difference, cause some waves to either cancel each other out (destructive interference or no amplitude) or reinforce each other (constructive interference or stronger amplitude). Conventional pigments produce the perceived color due to absorption, or the subtractive process, and reflection of the remaining light waves. In interference colors, the light waves which are reflected back from varying, small, distances interfere with each other and, depending on the wave phase difference, cause some waves to either cancel each other out (destructive interference or no amplitude) or reinforce each other (constructive interference or stronger amplitude).
 Therefore, the resultant color is dependent upon the degree to which the reflected rays are in or out of phase with each other which, in turn, is dependent upon the thickness of the metal oxide mica coating. In partial interference, some waves are reinforced while some are interfered with to produce the color. Therefore, the resultant color is dependent upon the degree to which the reflected rays are in or out of phase with each other which, in turn, is dependent upon the thickness of the metal oxide mica coating. In partial interference, some waves are reinforced while some are interfered with to produce the color.
In TiO2 coated mica, the perceived color (specular reflection) is determined by the TiO2 coating thickness ranging from silver (white), yellow (gold), red, blue, and green along with in-between colors (orange, violet, blue-green). As shown in the table, whichever color is reflected, the complimentary color is transmitted.
 Because of interference and transmission colors, pearlescent pigments contribute to a "flip/flop" effect. When applied on a white (all color reflecting) background, pearlescent coatings radiate both interference colors and transmission colors which vary with angle of illumination and observation and, thus, iridescence is exhibited. On a black color, the transmitted color would be absorbed. Because of interference and transmission colors, pearlescent pigments contribute to a "flip/flop" effect. When applied on a white (all color reflecting) background, pearlescent coatings radiate both interference colors and transmission colors which vary with angle of illumination and observation and, thus, iridescence is exhibited. On a black color, the transmitted color would be absorbed.
Examples of interference colors include our observation of prismatic or rainbow colors when viewing oil or gasoline spills on rainy wet streets, foam bubbles, or audio/video compact discs.
Earthtone and Gold Colors
The colors derived from Fe2O3 coated micas are both from the absorption effect (red iron oxide, yellow iron oxide) and the interference effect. The combination of the interference color and the oxide absorption color gives the following perceptible earth tone colors: yellow (bronze); orange (copper); red (russet); red (red russet), blue (blue russet). Here too the colors are determined by the coating thickness of the Fe2O3 layer ranging, with increasing thickness, from brass to copper to russet. A combination of TiO2 and Fe2O3 will produce the gold colors of light gold and gold.
Multi-Colors
Multicolor (three or more) effects are also possible with "combination pigments." Various absorption colors (iron oxides, chromium oxides, iron blue, carmine, or organic pigments) can be deposited onto the TiO2 coated mica and thus produce colors from absorption, interference, and transmission/reflection. A red absorption pigment coated on a blue reflecting interference pigment would reflect red diffusely, reflect blue spectrally, and, ultimately, reflect yellow which was initially transmitted as a compliment to the blue interference pigment. The yellow color transmitted would be reflected back from a white surface. Additionally, as a result of the red and yellow reflection, some orange might also be observed.
Upcoming back2basics Issues
In our next issue, we will continue our discussion of "effect pigments" by describing the optics involved with metallic (aluminum and bronze) pigments. The issue after that will focus on surfactants – chemistry, theory, and application in waterborne coatings and inks.
Contact Us
If we can be of any assistance with any of your "effect pigment" requirements, feel free to contact us at 914.273.0300.
|